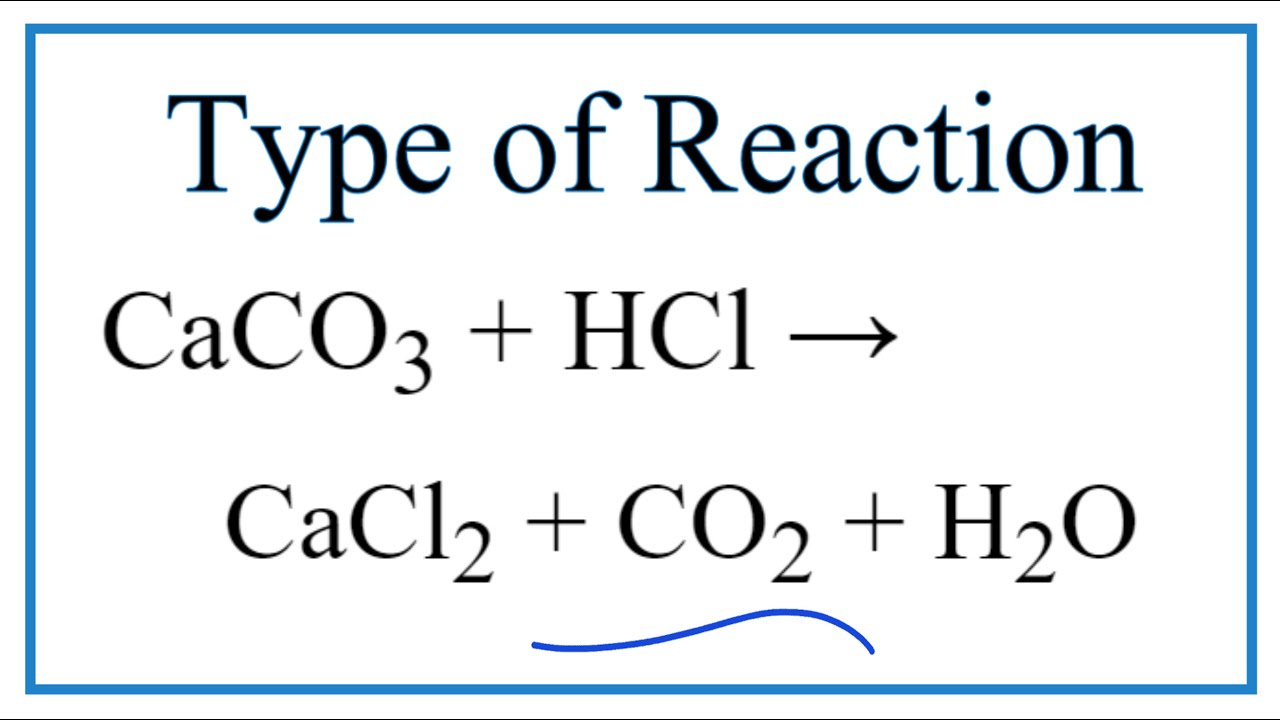
CaCO3 + 2HCl → CaCl2 + H2O + CO2 The mass of calcium chloride formed when 2.5 g of calcium carbonate is dissolved in excess of hydrochloric acid is:
To prepare carbon dioxide gas, 25.0 of calcium carbonate is added to a solution in which 20g hydrogen chloride has been dissolved. The unbalanced reaction is CaCo3 +HCl=CaCl2 +CO2+H2O…determine the limiting reagent? -

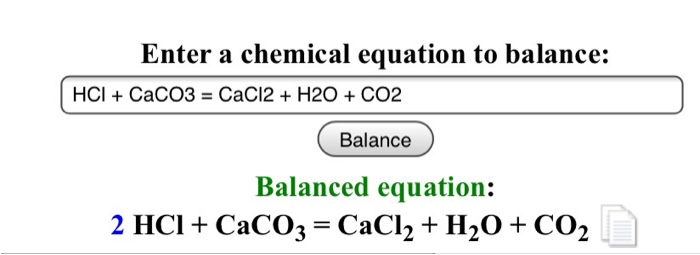
Balance the following equations : (a) Caco, (s) + HCl (aq) + CaCl, (aq) + H2O (1) + CO, (g) (b) Zn (s) + HCl - Brainly.in

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa

CaCO3+HCl=CaCl2+CO2+H2O Balanced Equation||Calcium Carbonate+Hydrochloric acid Balanced Equation - YouTube

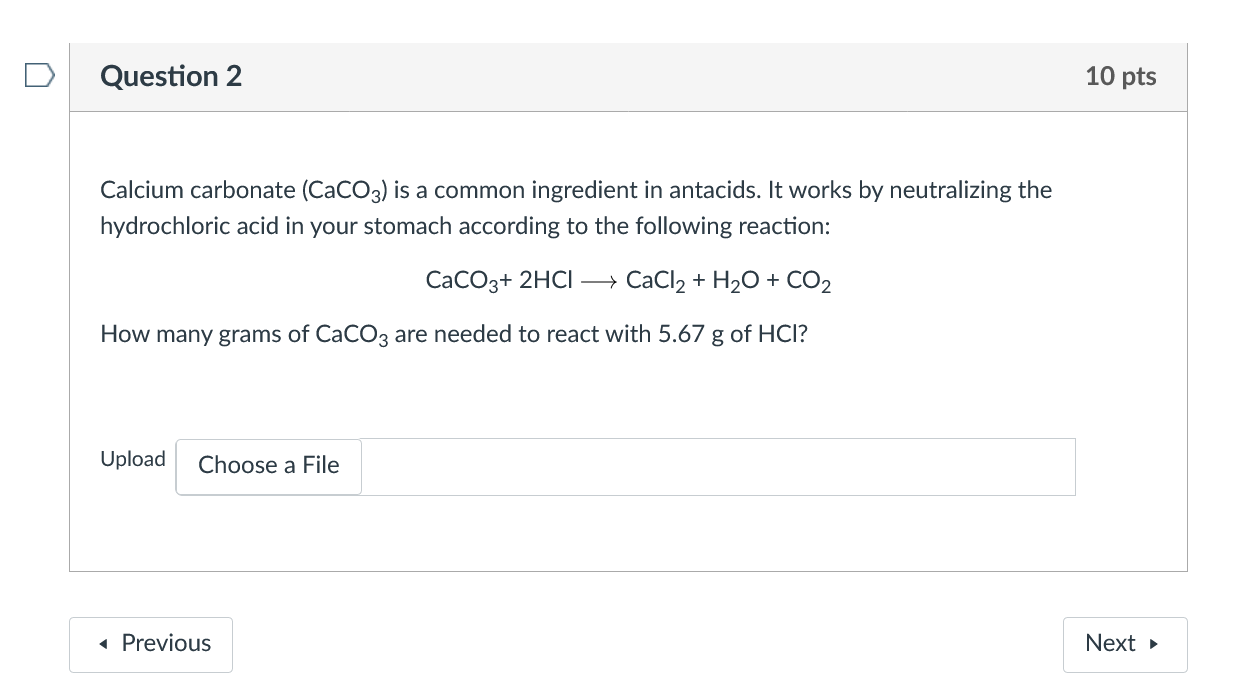
Calcium carbonate react with aqueous HCI to given below: CaCO3(s) + 2HCl (aq) → CaCl2 + CO2 + H2O What mass - Brainly.in

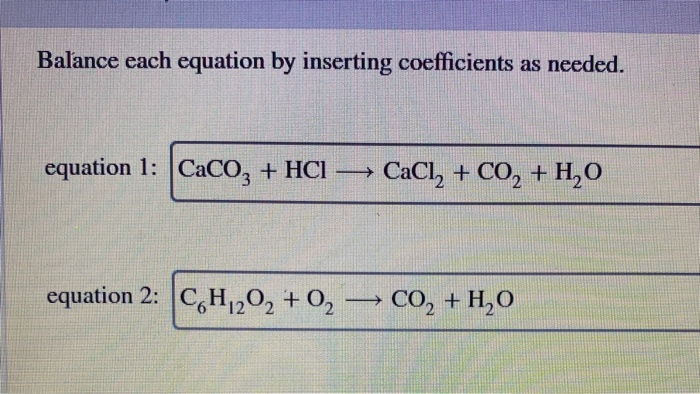
Balance the following equations: i) CaCO3 + HCl → CaCl2 + CO2 ↑ + H2O ii) Na + H2O → NaOH + H2 ↑ iii) (NH4)2 SO4 + Ca(OH)2 → CaSO4 +
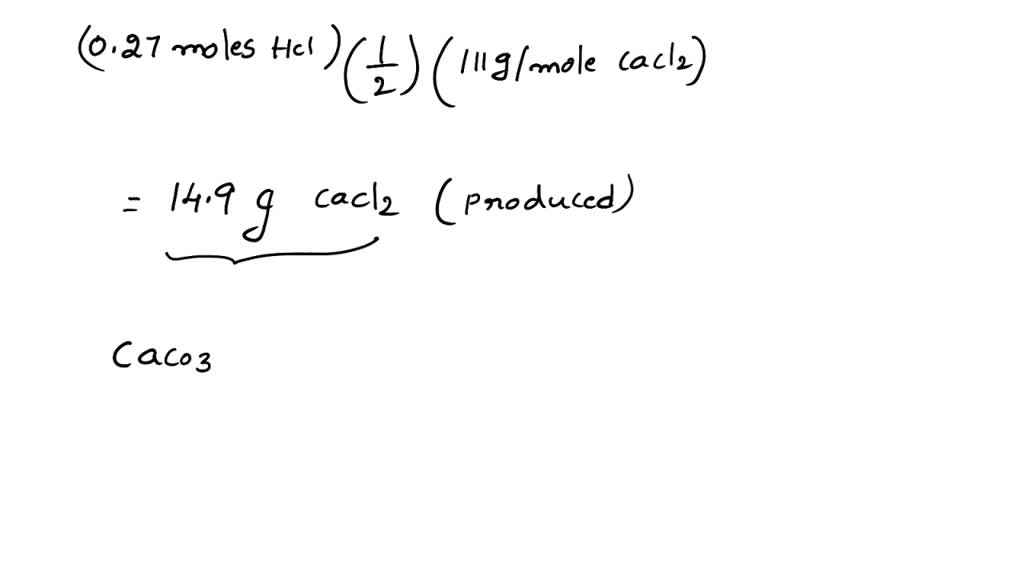
SOLVED: Calcium carbonate reacts with dilute hydrochloric acid. The equation for the reaction is shown: CaCO3 + 2HCl = CaCl2 + H2O + CO2. 1 g of calcium carbonate is added to
16. In a chemical reaction, caco3+2hcl= cacl2 +co2+h2o. 25ml hcl and 0.75M Calculate the amount of caco3
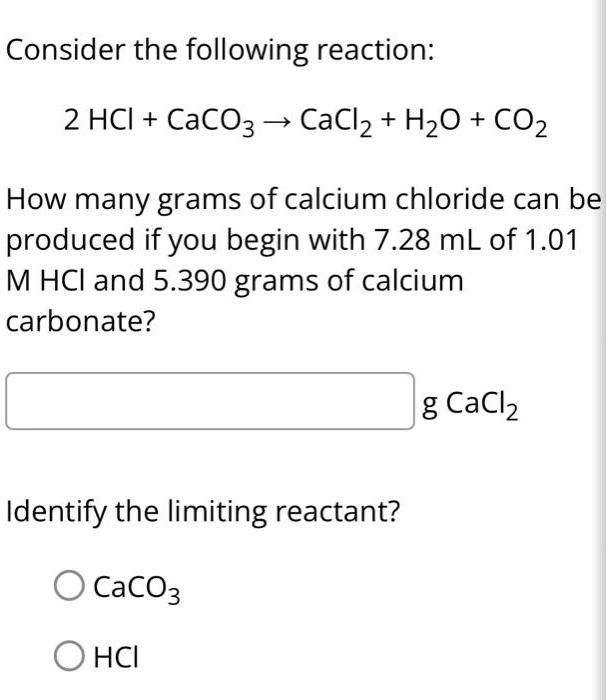
SOLVED: Consider the following reaction: 2 HCl + CaCO3 -> CaCl2 + H2O + CO2 How many grams of calcium chloride can be produced if you begin with 7.28 mL of 1.01







![Explain the reaction. CaCO3 + 2HCl⟶CaCl2 + H2O + CO2 [g ] Explain the reaction. CaCO3 + 2HCl⟶CaCl2 + H2O + CO2 [g ]](https://haygot.s3.amazonaws.com/questions/1352729_1287783_ans_adc66c29654147fb965de67e86f7f9a2.jpeg)