![CaCO3→ CaO + CO2,Δ H = + 180KJ . If entropies of CaCO3,CaO and CO2 respectively are 93,39 and 213J/mol/K , then Δ S (total) [in J/K] at 300K is CaCO3→ CaO + CO2,Δ H = + 180KJ . If entropies of CaCO3,CaO and CO2 respectively are 93,39 and 213J/mol/K , then Δ S (total) [in J/K] at 300K is](https://dwes9vv9u0550.cloudfront.net/images/10263170/3e36f846-ca64-4ea9-be05-3cab519e4454.jpg)
CaCO3→ CaO + CO2,Δ H = + 180KJ . If entropies of CaCO3,CaO and CO2 respectively are 93,39 and 213J/mol/K , then Δ S (total) [in J/K] at 300K is

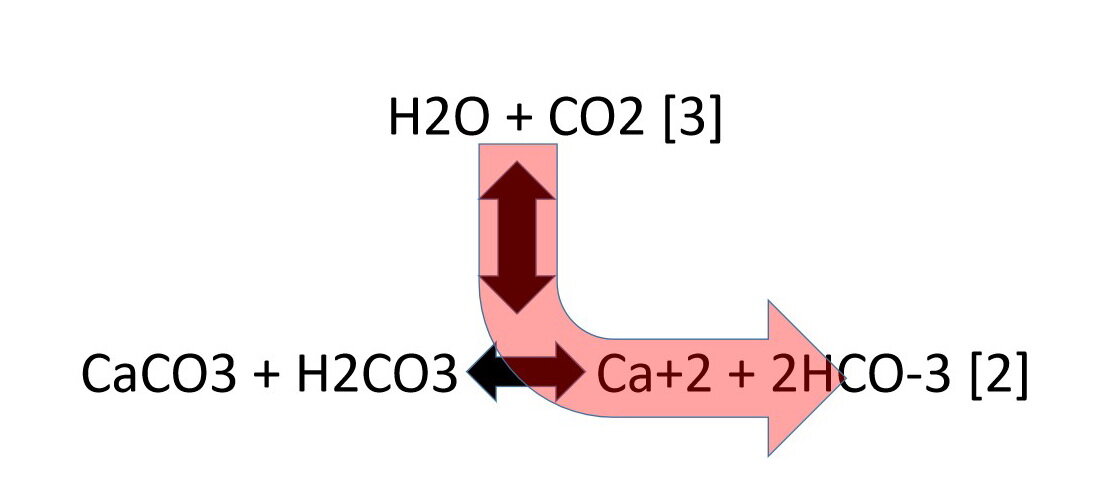
Summary of the reactions between carbon dioxide (CO2) with water (H2O)... | Download Scientific Diagram

SOLVED: A 100.0 g sample of impure calcium carbonate was heated. It decomposed to form carbon dioxide gas and calcium oxide. After heating, the solid residue weighed 78 grams. What was the

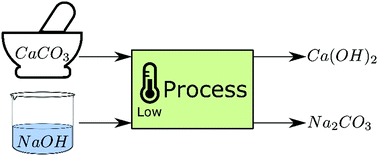
CO2 capture by aqueous Na2CO3 integrated with high-quality CaCO3 formation and pure CO2 release at room conditions - ScienceDirect
If 220 grams of calcium oxide (CaO) reacts with 50L of carbon dioxide (CO2), what mass of calcium carbonate (CaCO3) is produced? - Quora
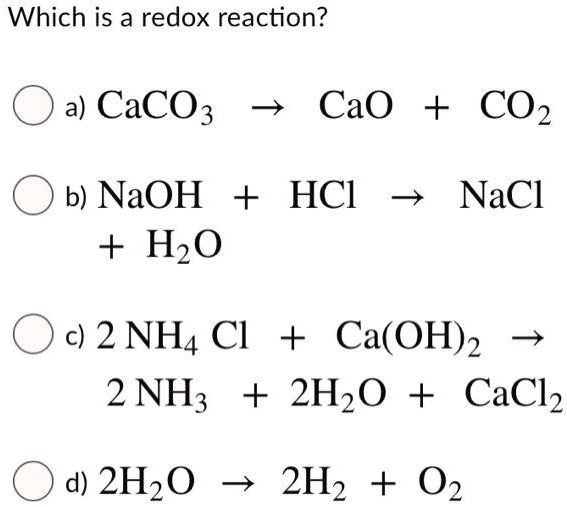
SOLVED: Which is a redox reaction? a) CaCO3 â†' CaO + CO2 b) NaOH + H2O â†' HCl + NaCl c) 2 NH4Cl + Ca(OH)2 â†' 2 NH3 + 2H2O + CaCl2 d) 2H2O â†' 2H2 + O2

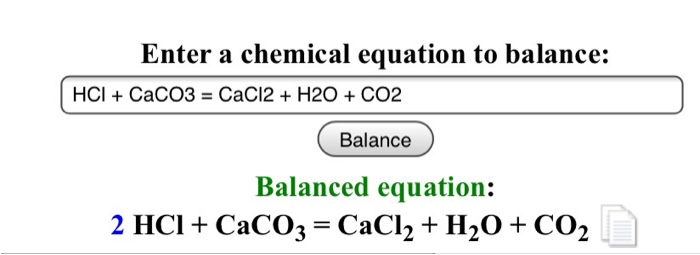
How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O | How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O Hey there! Are you struggling with balancing
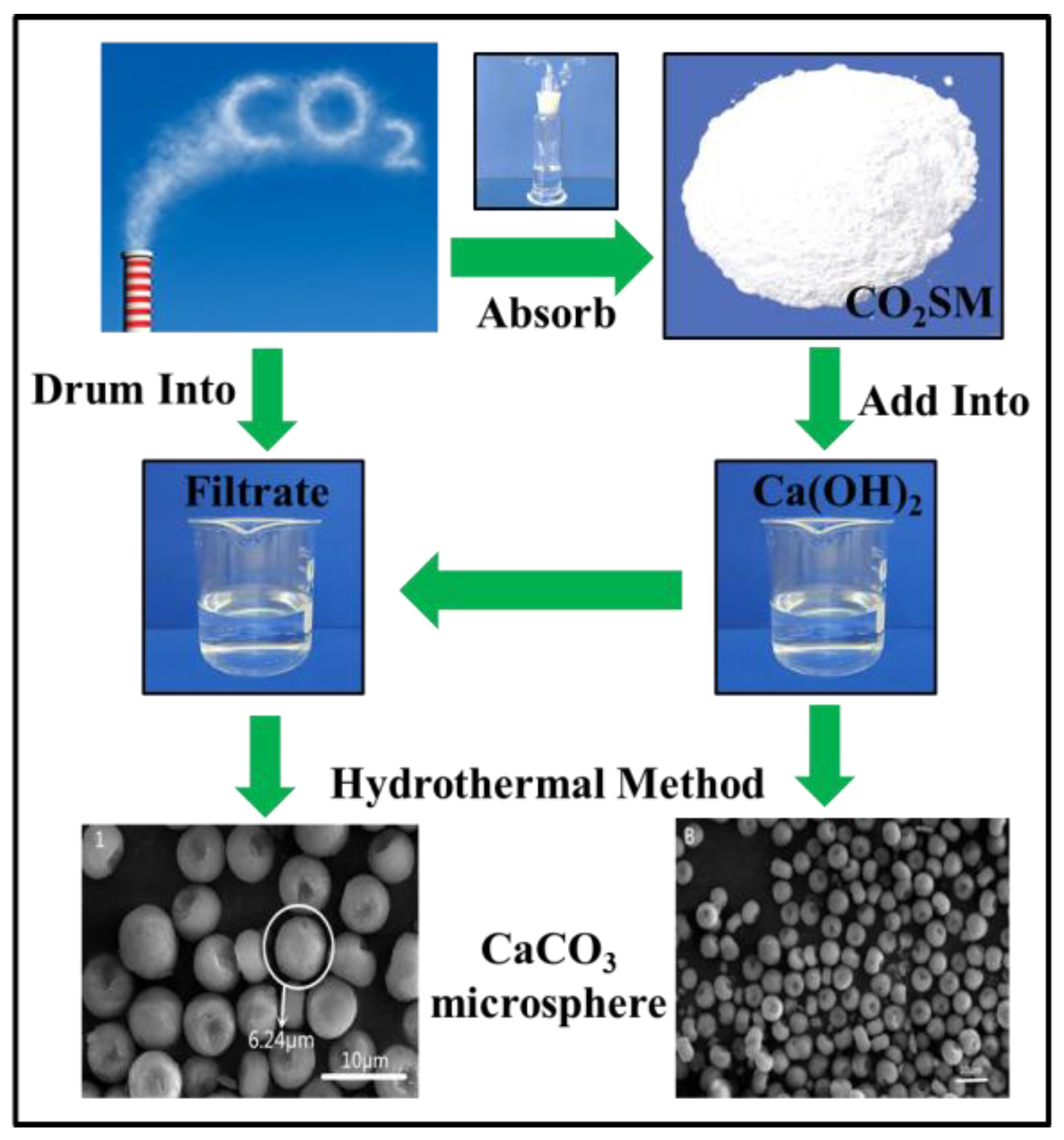
Crystals | Free Full-Text | Utilization of a CO2 Storage Material: Shape-Controlled Preparation of CaCO3 Microspheres

Question Video: Identifying the Chemical Equation- with State Symbols- That Corresponds to a Chemical Statement | Nagwa

Reaction steps involved in the formation of the CaCO3 sol from calcium... | Download Scientific Diagram
![CaCO3→ CaO + CO2,Δ H = + 180KJ . If entropies of CaCO3,CaO and CO2 respectively are 93,39 and 213J/mol/K , then Δ S (total) [in J/K] at 300K is CaCO3→ CaO + CO2,Δ H = + 180KJ . If entropies of CaCO3,CaO and CO2 respectively are 93,39 and 213J/mol/K , then Δ S (total) [in J/K] at 300K is](https://dwes9vv9u0550.cloudfront.net/images/2234632/d247a5dd-b799-4377-806f-2628447e070f.jpg)



![PDF] Energy analysis of CaCO3 calcination with CO2 capture | Semantic Scholar PDF] Energy analysis of CaCO3 calcination with CO2 capture | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/70ca22dce93c5e0c4e267a02ccc4e41951e44132/2-Figure1-1.png)